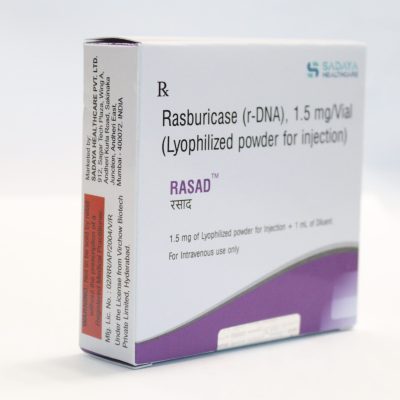
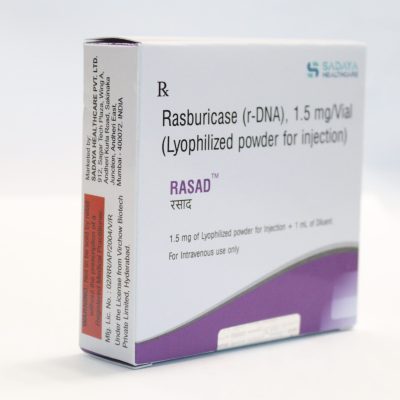
Brand name: Rasad
Marketed By: Sadaya Healthcare Pvt Ltd
Generic Name/API: Rasburicase (r-DNA)
Strength: 1.5 mg/Vial injection
Packaging: The 1.5 mg of lyophilized powder for injection + 1 ml of diluent.
Dosage: 0.5 mg intravenous injection
Pack size: One vial/pack
Strength: Injection of 1.5 mg
For more Info: Click here
A highly effective recombinant form of the enzyme urate oxidase, which catalyzes the oxidation of uric acid to allantoin. Allantoin is 5-10 times more soluble than uric acid, making excretion easier.
About Rasburicase
- Rasburicase is an antihyperuricemic drug.
- It belongs to the category of uricolytic agent.
- It is mainly used to treat acute hyperuricemia as a consequence of tumour lysis syndrome observed in patients undergoing chemotherapeutic therapy. In response to chemotherapy, a large number of cancer cells lyse within a fixed duration and consequently release large amounts of uric acid into the bloodstream. This condition is called hyperuricemia and can lead to renal failure and other health issues.
- Hyperuricemia can potentially induce uric acid crystallization leading to renal failure.
- Metabolism: The metabolism of purines to xanthine and then to uric acid in livers, is facilitated by the enzyme xanthine oxidase. Further metabolism of uric acid to allantoin takes place in most mammals except humans, due to lack of the enzyme urate oxidase. This loss is credited to a nonsense mutation in the coding region of the gene during hominoid evolution. Allantoin is 5- 10 times more soluble than uric acid, which makes excretion of the same more easier.
Strength:
- Each single-use vial containing 1.5 mg of rasburicase Injection and 3 ampules each containing 1.0 mL diluent solution for reconstitution, supplied in a 2 mL clear, glass ampule. It is composed of 1.0 mL sterile Water for Injection, USP, and 1.0 mg Poloxamer 188. The product reconstituted with diluent is a clear, colorless solution.
Recommended Dosage:
IV Preparation includes the following steps :
- Reconstitute with 1.5 mg vial w/ 1 mL and 7.5 mg vial w/ 5 mL of supplied diluent
- Mix by gently swirling; Do not shake or vortex
- Discard if discolored
- Further dilute dose in NS to a final volume of 50 mL.
- Reconstituted solution may be refrigerated but administered within 24 hr
- For IV Administration, infuse over 30 min.
- Do not administer bolus.
- Do not filter during infusion.
- If not possible to administer through a separate line, IV line should be flushed with at least 15 mL NS prior to and following rasburicase infusion.
- Initiate chemotherapy 4-24 hr after 1st dose.
- Discontinue if signs of hypersensitivity, hemolysis, or methemoglobinemia occur.
- Monitor plasma uric acid levels.
Warnings & Precautions:
- It is very important that your doctor checks you or your child while you are receiving this medicine to make sure the medicine is working properly and to decide if you should continue to receive it. Blood tests may be needed to check for unwanted effects.
- This medicine may cause a serious type of allergic reaction called anaphylaxis. Anaphylaxis can be life-threatening and requires immediate medical attention.
- Call your doctor right away if you or your child have a rash; itching; dizziness; lightheadedness; swelling of your hands, face, or mouth; trouble with breathing; or chest pain after you get the injection.
- Patients of African or Mediterranean ancestry are at higher risk of serious side effects and should be carefully evaluated by their doctor before starting this medicine.
- Check with your doctor right away if you or your child develop any of the following symptoms: bluish-colored lips, fingernails, or palms; dark urine; fever; headache; pale skin; rapid heart rate; shortness of breath; sore throat; or unusual bleeding or bruising after you receive this medicine.
Rasburicase side effects:
Side effects are unwanted symptoms caused by medicines. Even though all drugs cause side effects, not everyone gets them.
Serious
- Swelling of face, lips, tongue
- Wheezing and breathing problems
Common
- Nausea, vomiting
- Fever
- Swelling
- Anxiety
- Headache
- Abdominal pain
- Constipation
- Diarrhea
- Low phosphate levels in the blood
- High levels of liver enzymes in the blood
- Rashes
Storage and Handling:
- The lyophilized drug product and the diluent for reconstitution should be stored at 2-8oC (36-46oF).
- Do not freeze.
- Protect from light.
- Keep the medicine away from pets and children.
About Pharmacology
- Rasburicase, a recombinant urate oxidase, catalyzes the oxidation of uric acid to allantoin and hydrogen peroxide, bypassing the xanthine oxidase pathway. This reduces uric acid levels, preventing crystal formation in the kidneys and mitigating complications like renal impairment in conditions such as tumor lysis syndrome (TLS) and Lesch-Nyhan syndrome.
- After IV administration, rasburicase is distributed in extracellular fluid with a terminal half-life of 4.5 — 9.8 hours. It is primarily excreted unaltered via urine, and 60–70% of it is recovered within 24 hours. Clearance is reduced in cases of renal impairment, prolonging elimination and increasing drug exposure, which makes it important to adjust dosage.
Drug interactions
- Healthcare professionals should be informed in case a patient is using non-prescription or OTC medicines, alcohol or tobacco.
- Most metabolism-based drug interactions are not anticipated with these agents.There is no evidence of Rasburicase metabolising other cancer drugs like allopurinol, cytarabine, methylprednisolone, methotrexate, 6-mercaptopurine, thioguanine, etoposide, daunorubicin, cyclophosphamide or vincristine in vitro. However, allopurinol may interfere with the xanthine oxidase and reduce the concentrations of uric acid. This may reduce the effectiveness of rasburicase.
- Preclinical in vivo studies do not show any effect on the activity of isoenzymes CYP1A, CYP2A, CYP2B, CYP2C, CYP2E, and CYP3A, suggesting no induction nor inhibitory effect. With recommended dosage and schedule , no clinically relevant P450-mediated drug-drug interactions are therefore not anticipated in patients.
- For uric acid assay readings, a false low or negative reading may be observed due to the degradation of uric acid in blood/ plasma / serum samples at room temperature, by rasburicase. Hence, as a precaution blood should be collected in pre-chilled tubes with heparin, and immersed in an iced water bath, to prevent enzymatic activity.
- The plasma samples must be prepared by centrifugation in a pre-cooled centrifuge (4 °C) and maintained in an iced water bath and analyzed for uric acid within 4 hours of collection.
Important:
- Prior to initiating treatment, it is essential to engage in a comprehensive discussion with your healthcare provider regarding any current medications, medical conditions, pregnancy status, or intentions for breastfeeding. This dialogue allows your doctor to evaluate potential drug interactions and customize a treatment regimen that aligns with your specific requirements, emphasizing your safety and health. For personalized information and guidance, it is recommended to seek further clarification and advice from your doctor.
- The medicine is to be administered by a healthcare professional in a proper hospital setting, in the form of an injection (intravenous administration) over a time period of 30 minutes, once daily for up to 5 days. The dose, route of administration, and frequency will be decided by the physician based on your diagnosis.
Use in Specific Population
- Although studies done till date haven’t found any problem with respect to the below mentioned factors, consult a healthcare professional in case any specific issues arise:
- Allergies
- Pediatric
- Geriatric
- Rasburicase is proven safe and effective for children aged 1 month to 17 years in managing plasma uric acid levels in leukemia, lymphoma, and solid tumor patients undergoing chemotherapy. It was studied in 246 children, but data on infants under 6 months (n=7) was insufficient for comparison. Children under 2 years (n=24) had higher uric acid exposure and a lower success rate (83%) in normalizing levels by 48 hours compared to those aged 2–17 years (93%).
- Lack of adequate studies in breastfeeding women makes it important to weigh the benefits against risks for such patients. It is advised to wait for 2 weeks after the last dose to continue/ start breastfeeding. Alcohol may affect the potential efficacy of the drug and hence it is strongly recommended to avoid alcohol consumption while undergoing treatment with this drug. Effects of Rasburicase on lungs and liver are not known and hence, it is advised to consult a doctor in case of patients with underlying liver or lung diseases or disorders.
- It is unsafe to drive vehicles or operate heavy machinery after taking the injection because it contains a small amount of alcohol which may cause dizziness and impair your concentration.
Contraindications
- Anaphylaxis is one of the most common risks in this treatment, wherein hypersensitivity reactions can occur. In case of respiratory issues like bronchospasm, chest pain and tightness, dyspnea, hypoxia, hypotension, shock, and urticaria ,post treatment, immediately and permanently discontinue administration in any patient. Safety and efficacy have been established only for a single course of treatment once daily for 5 days. Hence, prolonged usage is not advisable without consulting a healthcare professional.
- Rasburicase can cause hemolysis especially in patients with G6PD deficiency. Hence, it is essential to screen patients for G6PD deficiency, especially those of African or Mediterranean ancestry; for G6PD deficiency. It can lead to methemoglobinemia as well.
- Cases of methemoglobinemia have resulted in severe hypoxemia requiring medical intervention. It is unclear whether individuals with cytochrome b5 reductase deficiency (previously called methemoglobin reductase deficiency) or other antioxidant enzyme deficiencies face a higher risk of methemoglobinemia or hemolytic anemia. If methemoglobinemia occurs, discontinue treatment immediately and permanently, and implement appropriate monitoring and supportive measures such as transfusion support or methylene blue administration.
Hemolytic reactions are often induced by hydrogen peroxide, produced in the process of conversion of uric acid to allantoin. Such reactions can occur within 2-4 days post the initiation of the therapy. It is highly advised to immediately and permanently discontinue therapy in such patients. - It is of utmost importance to keep the patient hydrated throughout the course of the treatment to ease the management of uric acid.
CT results and efficacy wrt comparator drug
- Data from three studies (n=265) analyzed plasma uric acid levels over time. Before treatment, 61 patients had uric acid ≥8 mg/dL, while 200 had <8 mg/dL. In those with uric acid ≥8 mg/dL (median 10.6 mg/dL), levels dropped by a median of 9.1 mg/dL within 4 hours of the first Rasburicase dose. In patients with uric acid <8 mg/dL (median 4.6 mg/dL), levels decreased by a median of 4.1 mg/dL within 4 hours. These results validate the efficacy of rasburicase in treating hyperuricemia.
Resources
Frequently Asked Questions


What is Rasburicase used for ?
Rasburicase is used to prevent or treat hyperuricemia (high levels of uric acid in the blood) in patients who are undergoing cancer chemotherapy for leukemia, lymphoma, and solid tumor malignancies. It is an important supportive therapy for patients undergoing chemotherapy to reduce the risk of complications associated with high levels of uric acid in the blood. It is often used prophylactically in individuals who are at high risk for TLS due to their cancer chemotherapy.
How should patients be monitored post administration of Rasburicase ?
Patients should be monitored for kidney function before and during treatment, with assessments such as serum cystatin C, urine dipstick, and urine protein-to-creatinine ratio. Regular monitoring for signs of hypersensitivity and allergic reactions is also recommended.
How is Rasburicase administered ?
The medicine will be administered to you by a healthcare professional in a hospital setting. Rasbase is given as an injection into a vein (intravenous infusion) over a period of 30 minutes, once a day for up to 5 days. The dose, route of administration, and frequency will be decided by your doctor based on your disease condition and other factors.
What are the common adverse reactions associated with Rasburicase ?
The most common adverse reactions (with an incidence greater than 20% and at least 5% higher than placebo) include upper respiratory tract infection, cough, pyrexia (fever), headache, arthralgia (joint pain), and oropharyngeal pain. If you experience any side effects consult your doctor immediately.
What is the cost of Rasburicase in India ?
The medication Rasburicase cost in India is relatively low because of the availability of generic brands. The Indian version of Rasburicase is more affordable compared to the innovator drug, Elitek. For information on its availability and current pricing, please contact SADAYA Healthcare.
How to Buy Rasburicase in India ?
To Buy Rasburicase online from India or inquire about its current price, you can contact SADAYA Healthcare directly or submit an enquiry through the portal.
What should be avoided during Rasburicase treatment ?
Usage of medication like allopurinol, another drug used to lower uric acid levels, is strongly discouraged as the general efficacy of rasburicase may be lowered.

Written By
Anonymous
Medical Content Writer

Approved By
Dr Anchal
Medical Advisor