Cyclophosphamide
Brand Name: Cylophos
Marketed By: Sadaya Healthcare Pvt Ltd
Strength: 200mg, 500mg, 1000mg/vial
Packaging: 200mg, 500mg, 1000mg
Pack Size: One vial/pack
More Information click here to see below CylophosDosage
FDA approved indications:
Cyclophosphamide is indicated for the treatment of:
- Malignant lymphomas (Stages III and IV of the Ann Arbor staging system), Hodgkin’s
disease, lymphocytic lymphoma (nodular or diffuse), mixed-cell type lymphoma,
histiocytic lymphoma, Burkitt’s lymphoma - Multiple myeloma
- Leukemias: chronic lymphocytic leukemia, chronic granulocytic leukemia (it is usually
ineffective in acute blastic crisis), acute myelogenous and monocytic leukemia, acute
lymphoblastic (stem-cell) leukemia (cyclophosphamide given during remission is
effective in prolonging its duration) - Mycosis fungoides (advanced disease)
- Neuroblastoma (disseminated disease)
- Adenocarcinoma of the ovary
- Retinoblastoma
Carcinoma of the breast
Mode of administration: - Intravenous administration (IV)
Mechanism of action:
- Cyclophosphamide is a prodrug, that means it requires some metabolite to activate itself
therapeutically. So, metabolic activation is mainly through the liver. An enzyme that plays a role
in activation of cyclophosphamide is mainly cytochrome P450 (CYP2B6). This enzyme helps to
convert inactive cyclophosphamide to its active form. Phosphoramide is one of the active
metabolites of cyclophosphamide which is mainly responsible for its anti-cancer activity. This
phosphoramide acts as an alkylating agent and adds an alkyl group to DNA, this addition will
lead to formation of cross link between DNA strands and prevents DNA replication. This cellular
process is interfered by cyclophosphamide and which proceeds for the cell death of cancer
cells.
Strength:
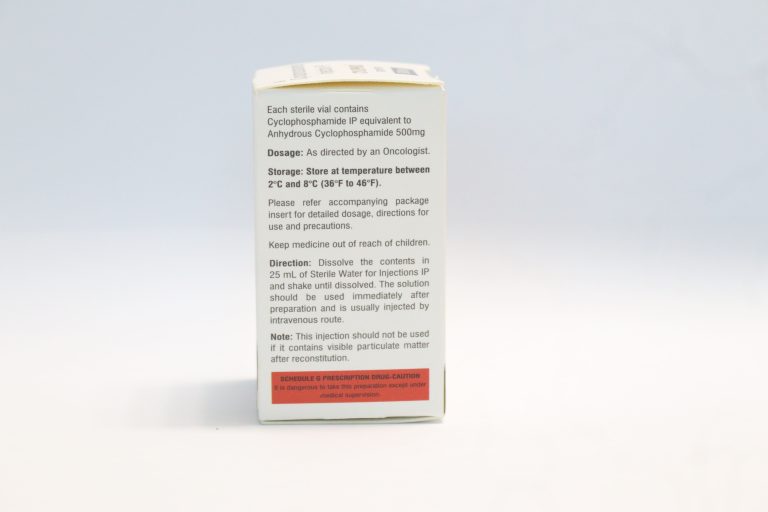
- It is available in 200, 500mg and 1000mg/ vial. It is a ready to dilute solution in a multiple dose
vial.
Recommended dosage:
- The starting dose recommended is 40-50mg/kg given as intravenous infusion and this treatment
cycle is for 2 – 5 days. Other Intravenous regimens include 10-15mg/kg for every 7 – 10 days or
else 3-5mg/kg twice weekly.
Warnings & precautions:
- Hematological monitoring is required as immunosuppression may lead to fatal infection.
- If a patient is suffering from any urinary tract infection, it should be cleared before
starting the treatment with cyclophosphamide. - Patients with a history of cardiac disease should consult a doctor before starting
cyclophosphamide. - Cyclophosphamide can cause fetal harm.
- Side effects of Cyclophosphamide:
Sore throat, fever, chills - Infection
- Shortness of breath
- Yellowing of eyes and skin
- Hairloss
- Loss of appetite
- Nausea
- Vomiting
- Abdominal pain
Storage and Handling:
- Store the vials in refrigerated condition at 2°C to 8°C (36°F to 46°F).
- Protect from light.
About Pharmacology
- Cyclophosphamide is an alkylating agent commonly used in cancer chemotherapy and
immunosuppressive therapy. It exerts its therapeutic effects by undergoing bioactivation in the
liver, where it is converted to its active metabolites, such as phosphoramide mustard, which
cross-links DNA strands, leading to DNA damage and inhibition of DNA replication and
transcription. This results in cell cycle arrest and eventual apoptosis, particularly in rapidly
dividing cells like those in tumors. - Cyclophosphamide exhibits an elimination half-life of 3 to 12 hours and total body clearance
(CL) values of 4 to 5.6 L/h. Following IV administration, its distribution is approximately 30 to 50
L, reflecting the volume of total body water, and about 20% of the drug is protein-bound. The
liver is the primary site of cyclophosphamide activation, involving cytochrome P450 enzymes
(CYP2A6, 2B6, 3A4, 3A5, 2C9, 2C18, and 2C19), with CYP2B6 exhibiting the highest
4-hydroxylase activity. Cyclophosphamide is metabolized to 4-hydroxycyclophosphamide, which
is in equilibrium with aldophosphamide. These can further metabolize to inactive metabolites or
undergo β-elimination to form active metabolites, phosphoramide mustard and acrolein. At high
doses, the 4-hydroxylation process decreases, leading to non-linear elimination.
Cyclophosphamide also induces its own metabolism, enhancing clearance and shortening the
half-life with repeated dosing. About 10 to 20% of the drug is excreted unchanged in the urine,
with 4% excreted in the bile.
Drug Interaction:
- Cyclophosphamide can interact with various medications, leading to enhanced toxicities.
Protease inhibitors may increase cytotoxic metabolites, raising the risk of infections and
neutropenia, while ACE inhibitors, natalizumab, paclitaxel, and others can exacerbate
hematotoxicity and immunosuppression. It can also increase cardiotoxicity when combined with
anthracyclines or trastuzumab, pulmonary toxicity with amiodarone or G-CSF, and
nephrotoxicity with amphotericin B or indomethacin. Other significant interactions include
increased hepatotoxicity with azathioprine, mucositis with protease inhibitors, and the risk of
thromboembolic events with tamoxifen. Cyclophosphamide may also lower cyclosporine levels,
increasing graft-versus-host disease risk, and its use with depolarizing muscle relaxants can
cause prolonged apnea. Careful monitoring is required when cyclophosphamide is used with
these agents.
Important:
- Before starting the treatment dose of cyclophosphamide, complete blood count of the
patient is essential to observe as it can help for dose adjustment can be done if required. - Patients who develop serious infection, cyclophosphamide treatment is not
recommended to such patients.
If a patient is having active urinary tract infection, use of cyclophosphamide should be
avoided.
Risk of cardiotoxicity can be increased with high doses of cyclophosphamide with a
previous or concomitant treatment with other cardiotoxic agents.
Monitoring of patients is required in case of pulmonary toxicity.
Use in specific population:
- Pediatric
- Geriatric
- Pregnancy and lactation
- Girls in late pre-pubescence are observed to develop ovarian fibrosis with complete loss of
germ cells; also there is a high risk of developing premature menopause after prolonged
cyclophosphamide treatment. While in pre-pubescent boys may have an increase in
gonadotropin secretion and may develop oligospermia (low sperm count) or azoospermia (a
condition in which there is no sperm in ejaculation). - Dose selection in case of elderly patients should be cautious.
There is a potential risk for pregnant women who are on treatment of cyclophosphamide as it
can cause fetal abnormalities and also can cause miscarriage issues. Lactating women are
advised not to breast feed during the cyclophosphamide treatment till 1st week of last dose.
Contraindications:
- In patients with a history of severe hypersensitivity reactions, cyclophosphamide is
contraindicated in them. Severe anaphylactic reactions may even cause death has been
reported. Cross-sensitivity with other alkylating agents can also occur. - Use of cyclophosphamide is contraindicated in patients suffering from urinary outflow
obstruction.
CT results and efficacy wrt comparator drug
- The study aimed to evaluate the effectiveness and toxicity of the combination of
cyclophosphamide and topotecan in pediatric patients with recurrent or refractory malignant
solid tumors. A total of 91 patients were treated, with 83 being fully assessable for response and
toxicity. The regimen consisted of cyclophosphamide (250 mg/m²/dose) followed by topotecan
(0.75 mg/m²/dose), administered as daily infusions for 5 days, alongside filgrastim to support
neutrophil recovery. - The results showed that the combination therapy was effective in treating rhabdomyosarcoma,
Ewing’s sarcoma, and neuroblastoma, with 10 of 15 patients with rhabdomyosarcoma, 6 of 17
with Ewing’s sarcoma, and 6 of 13 with neuroblastoma showing a complete or partial response.
Partial responses were observed in two of 18 patients with osteosarcoma and one patient with a
Sertoli-Leydig cell tumor. In total, 23 patients experienced either minor responses or stable
disease. - The treatment was generally well tolerated, with hematopoietic toxicity being the primary side
effect. Of 307 courses, 53% resulted in grade 3 or 4 neutropenia, 27% in grade 3 or 4 anemia,
and 44% in grade 3 or 4 thrombocytopenia. However, only 11% of courses were associated with
grade 3 or 4 infections. Non-hematopoietic toxicities were rare and mild, including nausea,
vomiting, mucositis, transaminase elevation, and hematuria. - In conclusion, the combination of cyclophosphamide and topotecan showed activity in treating
rhabdomyosarcoma, neuroblastoma, and Ewing’s sarcoma, with acceptable hematopoietic
toxicity when supported by filgrastim. While responses were limited in osteosarcoma, the
therapy remains a viable option for certain pediatric solid tumors.
1)https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212501s000lbl.pdf
2) https://ascopubs.org/doi/abs/10.1200/JCO.2001.19.15.3463
3)https://medlineplus.gov/druginfo/meds/a682080.html#:~:text=Before%20taking%20cyclo
phosphamide%2C&text=Your%20doctor%20may%20need%20to%20change%20the%2
0doses%20of%20your,you%20not%20to%20take%20cyclophosphamide.

FAQ
What are the indications in which Cylophos is used?
Malignant lymphoma
Multiple myeloma
Leukemia
Neuroblastoma
Adenocarcinoma of the ovary
Retinoblastoma
Carcinoma of the breast
What is the dose of Cylophos?
40-50mg/kg is the starting dose.
How do cylophos work in the body?
Cylophos activates after administration of it and its metabolites will prevent its DNA replication which will ultimately stop the cancer cell growth.
What are the precautions while administering cylophos?
Before starting the treatment for cyclophosphamide, the patient should tell the doctor if he/ she is allergic to it. If a patient is suffering from any urinary obstruction, then discontinue the cyclophos treatment.
Cylophos is a prescription medication?
Yes, Cyclophos is a prescription medication.

Written By
Ashwini Priya
Medical Content Writer

Approved By
Dr Anchal
Medical Advisor